When we study matter, we usually start by describing what we can see, feel, or measure directly. However, to truly understand how the universe works, we must look deeper than the surface. The chemical property definition refers to a characteristic of a substance that can only be observed during a chemical reaction. Unlike color or texture, these properties describe the potential of a substance to undergo a specific chemical change.
You cannot determine a chemical property just by looking at a sample in a jar. For example, looking at a gallon of gasoline does not tell you it is explosive; you only know that property when you expose it to a spark. This distinction is the cornerstone of chemistry. Whether you are a student memorizing periodic trends or an enthusiast experimenting at home, grasping this concept allows you to predict how different materials will interact with one another.
Physical Versus Chemical Properties
The most common stumbling block for students is confusing physical and chemical properties. To clear this up, we need to look at whether the substance changes its identity.
A physical property can be measured without changing the chemical composition of the substance. If you measure the boiling point of water, it turns from liquid to steam, but it is still H2O. If you tear a piece of paper, it is still paper.
In contrast, observing a chemical property requires the substance to become something else entirely. If you burn that same piece of paper, it turns into ash and smoke. You have observed the property of flammability, and the paper is gone forever.
Comparison Of Key Traits
- Observation Method: Physical properties are observed by inspection or physical measurement. Chemical properties are observed during a reaction.
- Result: Physical observation leaves the substance unchanged. Chemical observation results in new products.
- Reversibility: Physical changes are often reversible (like freezing water). Chemical changes are usually irreversible or require significant energy to reverse.
Major Examples Of Chemical Properties
There are many ways matter can react, but chemists classify these behaviors into specific categories. Here are the most important properties you will encounter in textbooks and laboratories.
Flammability
Flammability is the measure of how easily a substance burns. It is one of the most immediately dangerous and useful properties. When we say gasoline is flammable, we mean it reacts rapidly with oxygen to release heat and light. Materials like wood and paper have high flammability, while materials like steel or glass generally do not burn under normal conditions. This property dictates everything from building safety codes to rocket fuel engineering.
Toxicity
Toxicity describes the ability of a substance to damage a living organism. This is a chemical property because it involves the substance reacting with the complex chemistry of your body. For example, cyanide is toxic because it binds to iron in your blood cells, stopping them from carrying oxygen. This is a chemical reaction that happens inside biological systems.
Heat Of Combustion
This is a more technical term related to flammability. It quantifies exactly how much energy is released when a substance burns completely. Chemists and engineers use this property to determine which fuels are most efficient. Propane, for instance, has a high heat of combustion, making it excellent for heating homes and grilling food.
Reactivity With Water
Some substances are perfectly stable until they touch water. Sodium is a classic example. It is a soft metal, but if you drop a chunk of it into water, it reacts violently, producing hydrogen gas and heat, often causing an explosion. Knowing which elements are water-reactive is vital for laboratory safety and storage.
Oxidation States
Oxidation is the process of losing electrons. The most common example is iron reacting with oxygen to form rust. This property explains why some metals corrode quickly while others, like gold, remain shiny for thousands of years. Gold has a very low tendency to oxidize, which is considered a chemical property called chemical stability.
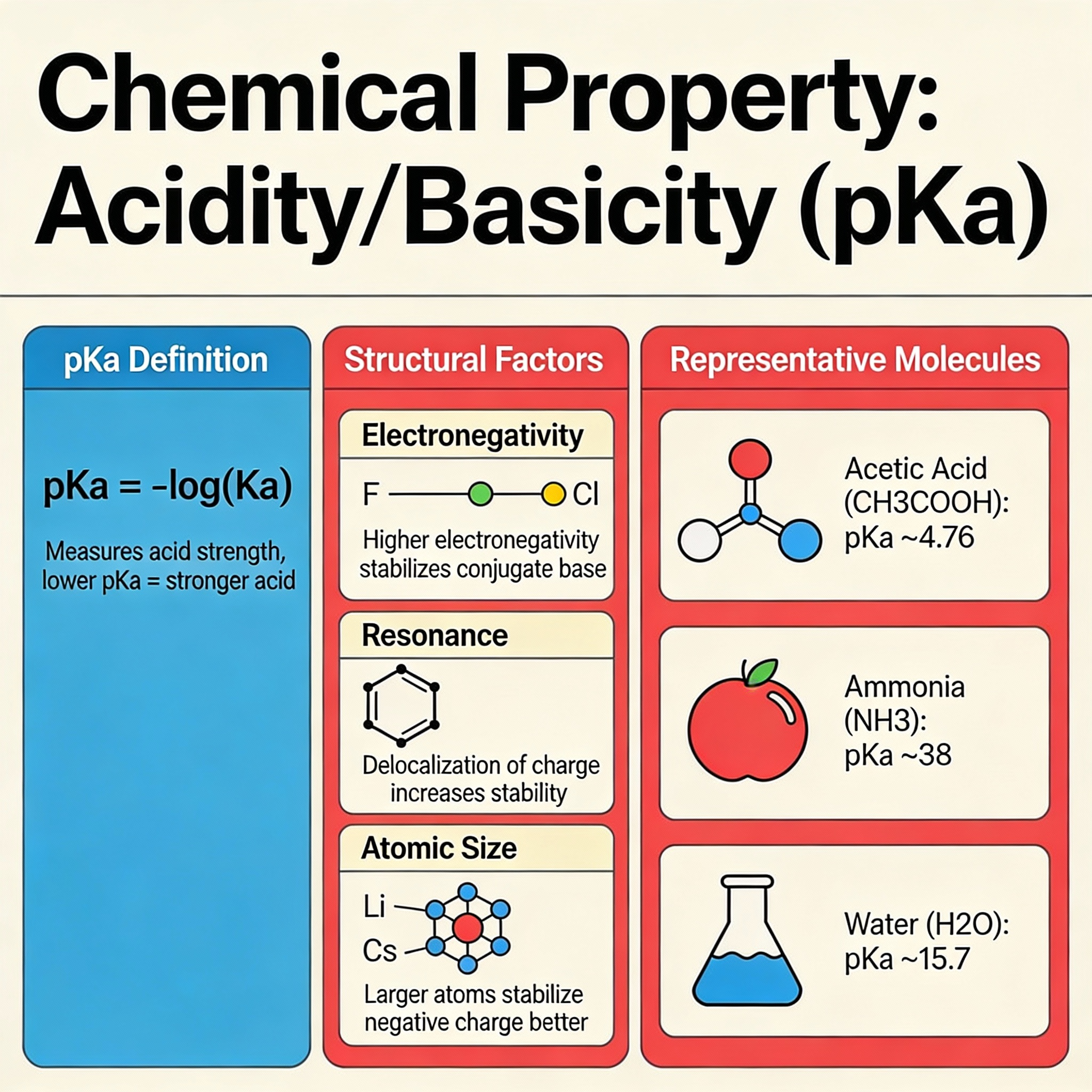
Acidity And Basicity
The pH scale is familiar to most people, but it is fundamentally a measure of chemical potential. Acidity and basicity describe how a substance donates or accepts protons (hydrogen ions) during a reaction.
Acids
Acids are substances that donate hydrogen ions. Examples include citric acid in lemons or sulfuric acid in car batteries. Their chemical property is their ability to dissolve metals or neutralize bases.
Bases
Bases are substances that accept hydrogen ions. Common examples are baking soda or bleach. They feel slippery to the touch because they react with the fatty acids in your skin, which is actually a mild chemical burn.
The Role Of Electrons
To understand why substances have these properties, we have to look at the atomic level. The behavior of an element is largely determined by its electrons, specifically the valence electrons. These are the electrons in the outermost shell of the atom.
Atoms want to be stable, which usually means having a full outer shell of electrons.
- Alkali Metals (Group 1) have one extra electron. They are desperate to get rid of it, which makes them highly reactive.
- Noble Gases (Group 18) have full outer shells. They do not want to gain or lose electrons, so they have the chemical property of being inert (non-reactive).
- Halogens (Group 17) are missing just one electron. They are aggressive in stealing electrons from other atoms, making them highly reactive oxidizers.
This electron behavior is why we can predict chemical properties using the Periodic Table. Elements in the same column often behave similarly because they have the same number of valence electrons.
Real World Applications
Understanding chemical properties is not just for passing exams. It shapes the world around us and informs critical decisions in industry and daily life.
Material Science
Engineers choose materials based on how they react with their environment. We build bridges out of steel but paint them to prevent the chemical property of rusting. We use stainless steel for cutlery because it resists reacting with the acids in food.
Medicine And Pharmacology
Developing drugs is entirely based on chemical properties. A pill must be stable enough to sit on a shelf but reactive enough to break down in your stomach and interact with specific cells in your body. If a drug is too reactive, it might damage healthy tissue (toxicity). If it is not reactive enough, it will not cure the disease.
Environmental Protection
Understanding how chemicals break down (or do not break down) helps us manage pollution. For example, plastic has the chemical property of being very stable and resistant to degradation. While this makes it a durable material, it also means it stays in the environment for centuries. Biodegradable plastics are designed with specific chemical weaknesses so nature can break them down faster.
Storage And Safety
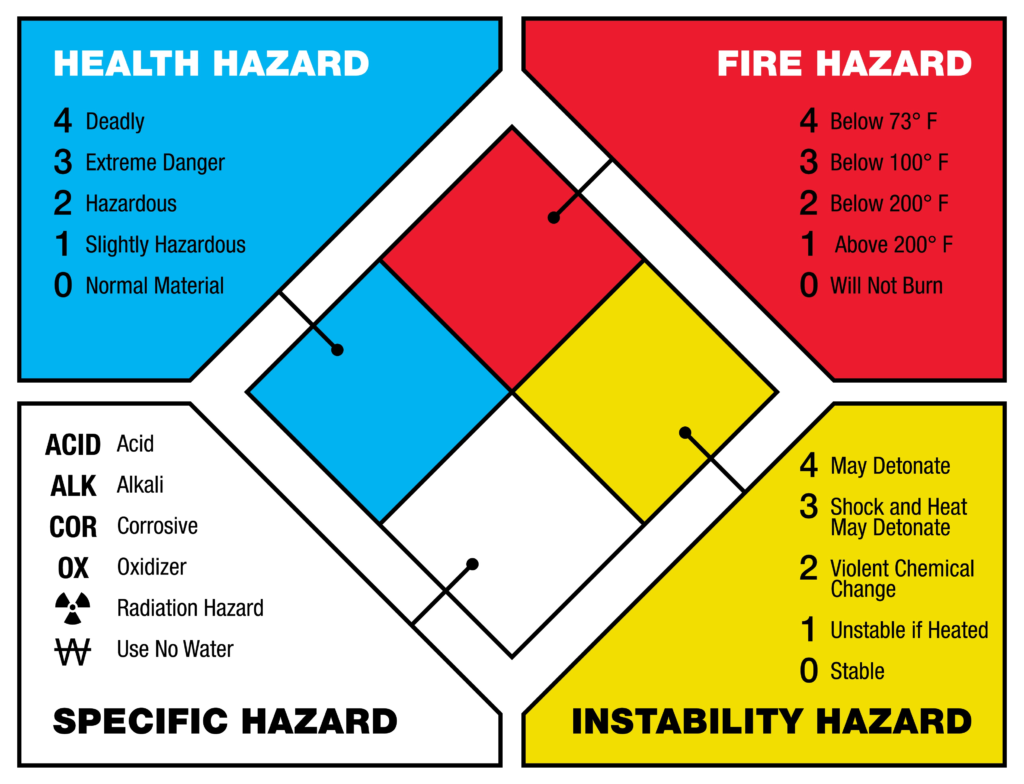
If you look at the back of a cleaning truck or a laboratory door, you will see diamond-shaped hazard signs. These symbols directly correspond to chemical properties like “Oxidizer,” “Corrosive,” or “Water Reactive.” These warnings save lives by ensuring firefighters and workers know exactly how to handle a spill or fire.
Conclusion
The chemical property definition gives us the language to describe how matter transforms. It moves us beyond simple observation and into the realm of prediction and innovation. By understanding traits like flammability, acidity, and reactivity, we gain control over the material world.
Next time you see a rusty nail, a burning candle, or a fizzing antacid tablet, remember that you are witnessing chemistry in action. These visible changes are the result of the hidden potential stored within the atoms themselves.