Chemistry is often compared to cooking, but unlike a pinch of salt here or a dash of spice there, chemistry requires absolute precision. Learning how to balance chemical equations is one of the most critical skills a student or science enthusiast must master. It is the mathematical representation of a chemical reaction, ensuring that the ingredients you put in perfectly match what comes out.
When you look at a reaction on paper, it might seem like a jumble of letters and numbers. However, once you understand the rules, it becomes a logical puzzle that is satisfying to solve. This guide will walk you through everything you need to know, from the basic definitions to advanced balancing techniques, ensuring you can tackle any homework problem or laboratory calculation with confidence.
The Law Of Conservation Of Mass
Before we start moving numbers around, we must understand the “why” behind the process. In nature, matter is neither created nor destroyed; it simply changes form. This fundamental principle is known as the Law of Conservation of Mass, attributed to the French chemist Antoine Lavoisier in the late 18th century.
In the context of a chemical equation, this means that the number of atoms of each element on the reactant side (the left) must exactly equal the number of atoms of that same element on the product side (the right). If you start with four carbon atoms, you must end with four carbon atoms, even if they are attached to different molecules. An unbalanced equation is scientifically incorrect because it implies that atoms have magically appeared or vanished into thin air.
Anatomy Of A Chemical Equation
To balance an equation effectively, you need to know the parts of the “sentence” you are reading. Let us break down the components.
Reactants And Products
- Reactants: These are the starting materials found on the left side of the arrow.
- Products: These are the new substances formed by the reaction, found on the right side.
- The Arrow: This symbol represents the change or “yields.” It shows the direction of the reaction.
Subscripts Versus Coefficients
This is the most important distinction to make. Confusing these two numbers is the most common reason students fail to balance equations correctly.
- Subscripts: These are the small numbers written after an element symbol (like the 2 in H2O). The subscript tells you how many atoms of that element are in a single molecule. You cannot change subscripts. Changing a subscript changes the identity of the substance entirely. For example, changing H2O to H2O2 turns water into hydrogen peroxide, which is definitely not something you want to drink.
- Coefficients: These are the large numbers written in front of a chemical formula (like the 2 in 2H2O). The coefficient tells you how many molecules of that substance are involved in the reaction. You can change coefficients. This is the only tool you have to balance the equation.
A Step-By-Step Method For Balancing
We recommend using the inspection method, often called the “ping-pong” method, for most standard equations. It involves going back and forth between reactants and products until everything matches.
Step 1: Write The Unbalanced Skeleton Equation
Start by writing down the formulas for your reactants and products. Do not add any coefficients yet. For example, let us look at the formation of water from hydrogen and oxygen. H2 + O2 -> H2O
Step 2: Take Inventory
List every element present in the reaction and count the current number of atoms for each side.
- Left Side: 2 Hydrogen, 2 Oxygen
- Right Side: 2 Hydrogen, 1 Oxygen
Step 3: Change Coefficients To Balance
Identify the element that is not balanced. In our example, Oxygen is unbalanced (2 on the left, 1 on the right). To fix this, we place a coefficient of 2 in front of the water molecule on the right. H2 + O2 -> 2H2O
Now, recount:
- Right Side: 2 x 2 = 4 Hydrogen, 2 x 1 = 2 Oxygen. Oxygen is fixed, but now Hydrogen is unbalanced (2 on the left, 4 on the right).
Step 4: Adjust And Recheck
Go back to the left side to fix the Hydrogen. We need 4 Hydrogen atoms, and we have H2. If we place a coefficient of 2 in front of the H2, we get: 2H2 + O2 -> 2H2O
Final Count:
- Left Side: 4 Hydrogen, 2 Oxygen
- Right Side: 4 Hydrogen, 2 Oxygen The equation is balanced.
Detailed Examples Of Balancing
Let us apply this logic to more complex scenarios. Seeing different types of reactions will help you recognize patterns.
Example 1: Combustion Of Methane
Combustion reactions are excellent for practice because they always involve a hydrocarbon reacting with oxygen to produce carbon dioxide and water. Skeleton: CH4 + O2 -> CO2 + H2O
- Inventory:
- Left: 1 C, 4 H, 2 O
- Right: 1 C, 2 H, 3 O (2 in CO2 + 1 in H2O)
- Carbon is balanced. Hydrogen is not.
- Balance Hydrogen: Place a 2 in front of H2O. CH4 + O2 -> CO2 + 2H2O
- Right Hydrogen is now 4. Hydrogen is balanced.
- Balance Oxygen: Recount the right side. We have 2 O (from CO2) + 2 O (from 2H2O) = 4 Oxygen total. On the left, we only have 2. Place a 2 in front of the O2. CH4 + 2O2 -> CO2 + 2H2O
- Final Check:
- Left: 1 C, 4 H, 4 O
- Right: 1 C, 4 H, 4 O Balanced.
Example 2: Photosynthesis
This is a classic biology equation that often intimidates students due to the larger numbers. Skeleton: CO2 + H2O -> C6H12O6 + O2
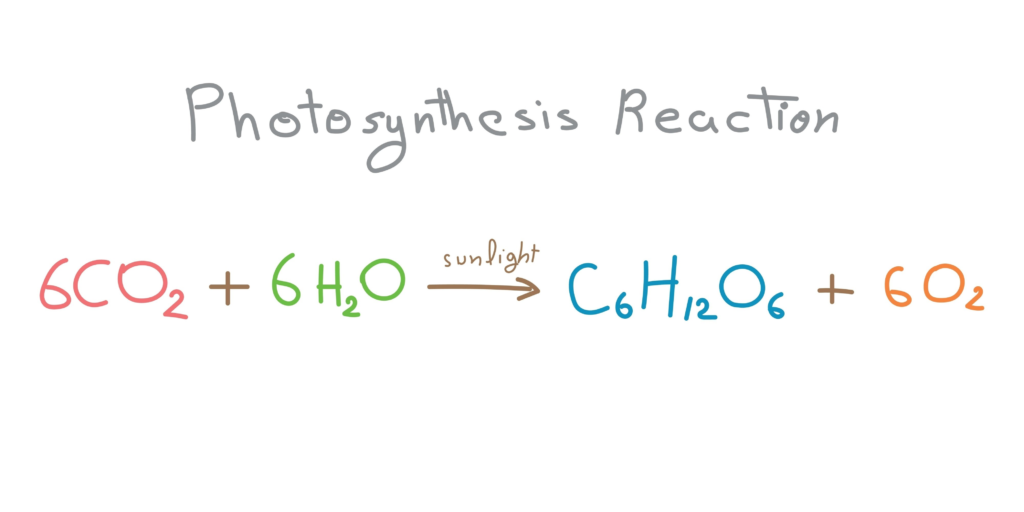
- Inventory:
- Left: 1 C, 2 H, 3 O
- Right: 6 C, 12 H, 8 O
- Balance Carbon: The glucose molecule has 6 Carbons. We need 6 on the left. 6CO2 + H2O -> C6H12O6 + O2
- Balance Hydrogen: The glucose molecule has 12 Hydrogens. We have 2 in water. Place a 6 in front of H2O. 6CO2 + 6H2O -> C6H12O6 + O2
- Balance Oxygen: This is the tricky part. Let us count the Oxygen on the left side first. (6 x 2) + (6 x 1) = 12 + 6 = 18 Oxygen atoms on the left. On the right, we have 6 inside glucose and 2 in the free Oxygen gas. We need 18 total. We already have 6 in glucose, so we need 12 more from the O2. To get 12 atoms from O2, we place a coefficient of 6. 6CO2 + 6H2O -> C6H12O6 + 6O2
- Final Check:
- Left: 6 C, 12 H, 18 O
- Right: 6 C, 12 H, 18 O Balanced.
Pro Tips For Difficult Equations
Sometimes the inspection method can feel like you are running in circles. Here are some strategies to break the loop.
Balance Polyatomic Ions As A Group
If you see a group of atoms like Sulfate (SO4), Nitrate (NO3), or Hydroxide (OH) appearing on both sides of the equation unchanged, treat the whole group as a single unit. For example, in a reaction involving Mg(NO3)2, instead of counting 1 Magnesium, 2 Nitrogens, and 6 Oxygens, simply count 1 Magnesium and 2 Nitrate groups. This saves massive amounts of time and reduces arithmetic errors.
Save Pure Elements For Last
If an element appears by itself (like O2, H2, or Fe) on one side of the equation, leave it for the very last step. Changing the coefficient of a pure element will not mess up the balance of any other elements, making it the easiest final adjustment.
Use Fractions Temporarily
Sometimes, the math works out that you need “half” a molecule to balance an equation. For example, if you need 7 Oxygen atoms and you have O2, you technically need 3.5 molecules. Write 3.5 to get the math right, and then multiply the entire equation by 2 at the very end to turn all coefficients into whole numbers. Chemists prefer whole numbers, but the fraction method is a great bridge to get there.
Common Pitfalls To Avoid
Even experienced chemists can make simple mistakes when rushing. Keep these common errors in mind.
- Changing Subscripts: We cannot stress this enough. Never touch the small numbers.
- Forgetting To Distribute: When you place a coefficient like 3 in front of Mg(OH)2, remember that it applies to the Mg, the O, and the H. It means 3 Magnesiums, 6 Oxygens, and 6 Hydrogens.
- Math Errors: Simple addition or multiplication mistakes are frequent. Always double-check your final count.
- Ignoring States of Matter: While they do not affect the math of balancing, writing (s), (l), (g), and (aq) is crucial for the scientific accuracy of the full equation.
Why This Skill Matters In The Real World
You might wonder if professional chemists actually sit around balancing equations all day. While computer programs can do this now, understanding the logic is essential for Stoichiometry. Stoichiometry is the calculation of reactants and products in chemical reactions.
If you are an industrial chemist creating fertilizer, you need to know exactly how much nitrogen to mix with hydrogen. If the equation is unbalanced, your calculations will be wrong. You might end up with unreacted chemicals that are wasted money or, worse, dangerous byproducts. In the pharmaceutical industry, precision is a matter of life and death.
Conclusion
Learning how to balance chemical equations is a rite of passage for every science student. It bridges the gap between the abstract concept of atoms and the tangible reality of laboratory experiments. By adhering to the Law of Conservation of Mass and following a systematic approach, you can solve even the most intimidating reaction puzzles.
Remember, practice is key. Start with simple synthesis reactions and work your way up to complex combustion and replacement reactions. With time, you will find that balancing becomes second nature, allowing you to focus on the fascinating chemical changes actually taking place.